Pharmacokinetics and Metabolism of Methylphenidate
August 15, 2015 at 02:12 PM | categories: genetics
Contents
1 Pharmacokinetics of Methylphenidate
Age at the time of drug treatment and pharmacokinetic differences in absorption, distribution and metabolism could influence both the acute and chronic effects of psychostimulants [28]. For a 10 mg dose in a 30 kg child, the maximum serum concentration occurs about 1.5 to 2 hours afterward and leads to concentrations of approximately 10 ng/mL in plasma after administration, dropping by 50 % about 2 hours later [4]. However, the optimal therapeutic dose varies considerably across individuals [9]. There is great variability among subjects and among dosing regimens in the rate of absorption of methylphenidate (MPH) and the rate of decay of MPH from plasma. This led to a 3.5-fold difference in blood levels between subjects taking the same dose in the most extreme case [21]. The therapeutic effects parallel the serum concentration of immediate-release MPH, with a maximum reduction in attention-deficit/hyperactivity disorder (ADHD) symptoms about 1.5 to 2 hours after dosing followed by a decline that is sufficient to require another dose about 4 hours after the first to reestablish full efficacy [23].
Oral MPH in normal adults has a half-life of about 2.1 h, the time to peak plasma concentration is about 2.2 h. Ritalinic acid (major metabolite) reaches peak plasma concentration at approximately the same time as MPH except that levels were several-fold those of MPH [27]. Oral MPH in ADHD children isn’t too different: half-life 2.4 h; time to peak 1.5 h [27].
MPH undergoes extensive and stereospecific presystemic metabolism [1] to form predominantly the inactive hydrolysis product, ritalinic acid, resulting in a low absolute oral bioavailability. The bioavailability of oral MPH measured in rat is 0.19, in monkey 0.22, with considerable intersubject variability: ranging from 0.08 to 0.44 in both species [27]. Animal studies have suggested that MPH is subject to substantial first-pass and presystemic metabolism, the primary action of presystemic metabolism is deesterification in the gut and/or intestinal wall [1, 27]. Interestingly, the rate and extent of d-MPH absorption were similar when administered with or without food [22]. MPH is metabolized extensively in the rat; less than 1 % unchanged MPH was found in the 48 h urine [7].
Intravenous administration of 1 mg/kg of MPH in rats shows rapid accumulation of MPH in the brain, the peak brain concentration within the first minute after injection [13]. Transfer of MPH from plasma to tissues is rapid and distribution is extensive [7]. During the first 30 min after MPH administration, there was an average of 8-fold greater MPH concentration in brain vs. plasma [13]. Peak serum and brain concentrations after oral administration of 1 mg/kg of MPH occurred at 10 min after dosing. At 45 min after dosing, the brain/serum ratios were approximately equal for both i.v. and oral routes of administration [13]. The half-life of MPH in the rat is 24.8 min [7]. MPH is ionized at physiological pH to a lesser extent than dextroamphetamine (D-AMPH) and is considerably more lipid soluble, which will induce quicker rise times to the brain [7].
After oral administration of [11C]d-threo-MPH, the radiolabeled d-MPH was distributed stereoselectively in the rat brain, especially in the striatum. Further, it was shown that the d-isomer binds specifically to dopamine (DA) uptake sites in the striatum [2]. [11C]l-threo-MPH had an homogeneous distribution throughout the brain (after i.v. administration) [26]. The rate of uptake in the brain for the two enantiomers was eqivalent but the clearance was significantly slower for [11C]d-threo-MPH than for the l-isomer. The therapeutic and safety profile of d-MPH is similar to that of racemic d,l-MPH [15]. However, l-MPH offers anxiolytic and antipsychotic activity, serves as an antidote for stimulant overdose [3], reduces cocaine induced locomotor sensitization [6], and pretreatment with l-MPH attenuates the motor activity response to d-MPH [5].
The median effective dose (ED50) for dopamine transporter (DAT) blockade was
calculated to be 0.25 mg/kg oral MPH [24] and 0.075 mg/kg i.v. MPH [25]; the half
maximal inhibitory concentration (IC50) of MPH at DAT: 84 nm [8]. The ED50 estimate
for global norepinephrine transporter (NET) was 0.14 mg/kg ± 0.02 mg/kg MPH,
although the local ED50 did vary by brain region [10]; the IC50 of MPH at NET:
510 nm [8] If occupancy = 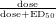 [10], then the average efficacious maintenance
doses of MPH used in children and adults occupy 70 % to 80 % of NET but only 60 %
to 70 % of DAT, based on the estimated ED50 value of 0.14 mg/kg for NET and
0.25 mg/kg for DAT [24]. Interestingly, NET has greater affinity for DA than for
norepinephrine (NE) [12], and whether DAT or NET is the predominant protein
clearing DA depends on the abundance of the two transporters in a given region
[11].
[10], then the average efficacious maintenance
doses of MPH used in children and adults occupy 70 % to 80 % of NET but only 60 %
to 70 % of DAT, based on the estimated ED50 value of 0.14 mg/kg for NET and
0.25 mg/kg for DAT [24]. Interestingly, NET has greater affinity for DA than for
norepinephrine (NE) [12], and whether DAT or NET is the predominant protein
clearing DA depends on the abundance of the two transporters in a given region
[11].
1.1 Sustained-release preparations of MPH
In recent years, new drug delivery methods have been developed to enhance the duration of action [21]. Through these efforts to develop effective long-acting MPH preparations, valuable new information has become available on the relationship between dosing regimens, blood levels, and therapeutic response. Efficacy of MPH declined in an administration regimen designed to provide consistent stable blood levels, presumably due to acute tolerance due to an adaptation response at the synaptic level in response to blockade of the DAT [18, 20]. This confirmed and extended findings of rapid tolerance previously observed by Srinivas et al. [16] in an acute administration paradigm. This lead to the discovery that there are two alterative strategies for providing sustained efficacy: Pulsatile delivery or escalating blood levels [17]. With pulsatile delivery, there is a drop in blood levels that enables the system to retain or regain sensitivity. With escalating delivery, the increasing blood levels presumably help to overcome emerging tolerance and waning sensitivity [19].
1.2 Placebo effects of MPH
Subjects receiving placebo showed no significant degree of benefit and on average were slightly worse following placebo administration than when they first arrived at the laboratory in an unmedicated state [21]. ADHD does not appear to be a placebo-responsive disorder when evaluated using objective measures. Instead, after placebo administration, a clear pattern of deterioration across the day emerges, in contrast to the pattern of improvement with MPH [18]. Improvement of ADHD children on placebo in studies using observer ratings is probably due in large part to a halo effect, in which teachers or parents score children differently if they believe that they have been medicated [21]. Also, when MPH is given therapeutically and produces large improvements in the classroom, children with ADHD do not attribute this ‘success’ to the drug [14]. This indicates that expectancy does not play a prominent role in MPH’s therapeutic effects.
Acronyms
- ADHD
- attention-deficit/hyperactivity disorder
- D-AMPH
- dextroamphetamine
- DAT
- dopamine transporter
- DA
- dopamine
- ED50
- median effective dose
- IC50
- half maximal inhibitory concentration
- MPH
- methylphenidate
- NET
- norepinephrine transporter
- NE
- norepinephrine
References
[1] T. Aoyama, H. Kotaki, and T. Iga. Dose-dependent kinetics of methylphenidate enantiomers after oral administration of racemic methylphenidate to rats. J Pharmacobiodyn, 13(10):647–52, Oct 1990.
[2] T. Aoyama, H. Kotaki, Y. Sawada, and T. Iga. Stereospecific distribution of methylphenidate enantiomers in rat brain: specific binding to dopamine reuptake sites. Pharm Res, 11(3):407–11, Mar 1994.
[3] R. Baldessarini and A. Campbell. Method of dopamine inhibition using l-threo-methylphenidate, Apr. 2001. US Patent 6,221,883.
[4] Y. P. Chan, J. M. Swanson, S. S. Soldin, J. J. Thiessen, S. M. Macleod, and W. Logan. Methylphenidate hydrochloride given with or before breakfast: Ii. effects on plasma concentration of methylphenidate and ritalinic acid. Pediatrics, 72(1):56–9, Jul 1983.
[5] E. Davids, K. Zhang, N. S. Kula, F. I. Tarazi, and R. J. Baldessarini. Effects of norepinephrine and serotonin transporter inhibitors on hyperactivity induced by neonatal 6-hydroxydopamine lesioning in rats. J Pharmacol Exp Ther, 301(3):1097–102, Jun 2002.
[6] Y.-S. Ding, S. J. Gatley, P. K. Thanos, C. Shea, V. Garza, Y. Xu, P. Carter, P. King, D. Warner, N. B. Taintor, D. J. Park, B. Pyatt, J. S. Fowler, and N. D. Volkow. Brain kinetics of methylphenidate (ritalin) enantiomers after oral administration. Synapse, 53(3):168–75, Sep 2004. doi: 10.1002/syn.20046.
[7] J. Gal, B. J. Hodshon, C. Pintauro, B. L. Flamm, and A. K. Cho. Pharmacokinetics of methylphenidate in the rat using single-ion monitoring glc-mass spectrometry. J Pharm Sci, 66(6):866–9, Jun 1977.
[8] S. J. Gatley, N. D. Volkow, A. N. Gifford, J. S. Fowler, S. L. Dewey, Y. S. Ding, and J. Logan. Dopamine-transporter occupancy after intravenous doses of cocaine and methylphenidate in mice and humans. Psychopharmacology (Berl), 146(1):93–100, Sep 1999.
[9] L. L. Greenhill, J. M. Swanson, B. Vitiello, M. Davies, W. Clevenger, M. Wu, L. E. Arnold, H. B. Abikoff, O. G. Bukstein, C. K. Conners, G. R. Elliott, L. Hechtman, S. P. Hinshaw, B. Hoza, P. S. Jensen, H. C. Kraemer, J. S. March, J. H. Newcorn, J. B. Severe, K. Wells, and T. Wigal. Impairment and deportment responses to different methylphenidate doses in children with adhd: the mta titration trial. J Am Acad Child Adolesc Psychiatry, 40(2):180–7, Feb 2001. doi: 10.1097/ 00004583-_200102000-_00012.
[10] J. Hannestad, J.-D. Gallezot, B. Planeta-Wilson, S.-F. Lin, W. A. Williams, C. H. van Dyck, R. T. Malison, R. E. Carson, and Y.-S. Ding. Clinically relevant doses of methylphenidate significantly occupy norepinephrine transporters in humans in vivo. Biol Psychiatry, 68(9): 854–60, Nov 2010. doi: 10.1016/j.biopsych.2010.06.017.
[11] J. A. Morón, A. Brockington, R. A. Wise, B. A. Rocha, and B. T. Hope. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci, 22(2):389–95, Jan 2002.
[12] T. Pacholczyk, R. D. Blakely, and S. G. Amara. Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature, 350(6316):350–4, Mar 1991. doi: 10.1038/350350a0.
[13] K. S. Patrick, K. R. Ellington, and G. R. Breese. Distribution of methylphenidate and p-hydroxymethylphenidate in rats. J Pharmacol Exp Ther, 231(1):61–5, Oct 1984.
[14] W. E. Pelham, B. Hoza, H. L. Kipp, E. M. Gnagy, and S. T. Trane. Effects of methylphenidate and expectancy of ADHD children’s performance, self-evaluations, persistence, and attributions on a cognitive task. Exp Clin Psychopharmacology, 5:3–13, 1997.
[15] D. Quinn, S. Wigal, J. Swanson, S. Hirsch, Y. Ottolini, M. Dariani, M. Roffman, J. Zeldis, and T. Cooper. Comparative pharmacodynamics and plasma concentrations of d-threo-methylphenidate hydrochloride after single doses of d-threo-methylphenidate hydrochloride and d,l-threo-methylphenidate hydrochloride in a double-blind, placebo-controlled, crossover laboratory school study in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry, 43(11):1422–9, Nov 2004. doi: 10.1097/ 01.chi.0000140455.96946.2b.
[16] N. R. Srinivas, J. W. Hubbard, D. Quinn, and K. K. Midha. Enantioselective pharmacokinetics and pharmacodynamics of dl-threo-methylphenidate in children with attention deficit hyperactivity disorder. Clin Pharmacol Ther, 52(5):561–8, Nov 1992.
[17] J. Swanson, S. Gupta, A. Lam, I. Shoulson, M. Lerner, N. Modi, E. Lindemulder, and S. Wigal. Development of a new once-a-day formulation of methylphenidate for the treatment of attention-deficit/hyperactivity disorder: proof-of-concept and proof-of-product studies. Arch Gen Psychiatry, 60(2):204–11, Feb 2003.
[18] J. M. Swanson, S. Gupta, L. Williams, D. Agler, M. Lerner, and S. Wigal. Efficacy of a new pattern of delivery of methylphenidate for the treatment of ADHD: effects on activity level in the classroom and on the playground. J Am Acad Child Adolesc Psychiatry, 41:1306–1314, 2002.
[19] J. M. Swanson, B. J. Casey, J. Nigg, F. X. Castellanos, N. D. Volkow, and E. Taylor. Clinical and cognitive definitions of attention deficits in children with attention-deficit/hyperactivity disorder. In M. I. Posner, editor, Cognitive neuroscience of attention, pages 430– 445. Guilford, New York, NY, 2004.
[20] J. L. Swanson-Park, C. M. Coussens, S. E. Mason-Parker, C. R. Raymond, E. L. Hargreaves, M. Dragunow, A. S. Cohen, and W. C. Abraham. A double dissociation within the hippocampus of dopamine d1/d5 receptor and beta-adrenergic receptor contributions to the persistence of long-term potentiation. Neuroscience, 92(2):485–97, 1999.
[21] M. H. Teicher, A. Polcari, M. Foley, E. Valente, C. E. McGreenery, W.-W. Chang, G. McKay, and K. K. Midha. Methylphenidate blood levels and therapeutic response in children with attention-deficit hyperactivity disorder: I. effects of different dosing regimens. J Child Adolesc Psychopharmacol, 16(4):416–31, Aug 2006. doi: 10.1089/cap.2006.16.416.
[22] S. K. Teo, M. R. Scheffler, A. Wu, D. I. Stirling, S. D. Thomas, D. Stypinski, and V. D. Khetani. A single-dose, two-way crossover, bioequivalence study of dexmethylphenidate hcl with and without food in healthy subjects. J Clin Pharmacol, 44(2):173–8, Feb 2004. doi: 10.1177/0091270003261899.
[23] N. D. Volkow and J. M. Swanson. Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiatry, 160(11):1909–1918, Nov 2003.
[24] N. D. Volkow, G. J. Wang, J. S. Fowler, S. J. Gatley, J. Logan, Y. S. Ding, R. Hitzemann, and N. Pappas. Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry, 155(10):1325–1331, Oct 1998.
[25] N. D. Volkow, G. J. Wang, J. S. Fowler, M. Fischman, R. Foltin, N. N. Abumrad, S. J. Gatley, J. Logan, C. Wong, A. Gifford, Y. S. Ding, R. Hitzemann, and N. Pappas. Methylphenidate and cocaine have a similar in vivo potency to block dopamine transporters in the human brain. Life Sci, 65(1):PL7–12, 1999.
[26] N. D. Volkow, J. S. Fowler, G.-J. Wang, Y. S. Ding, and S. J. Gatley. Role of dopamine in the therapeutic and reinforcing effects of methylphenidate in humans: results from imaging studies. European Neuropsychopharmacology, 12:557–566, 2002.
[27] W. Wargin, K. Patrick, C. Kilts, C. T. Gualtieri, K. Ellington, R. A. Mueller, G. Kraemer, and G. R. Breese. Pharmacokinetics of methylphenidate in man, rat and monkey. J Pharmacol Exp Ther, 226(2): 382–6, Aug 1983.
[28] P. B. Yang, A. C. Swann, and N. Dafny. Dose-response characteristics of methylphenidate on locomotor behavior and on sensory evoked potentials recorded from the vta, nac, and pfc in freely behaving rats. Behav Brain Funct, 2:3, 2006. doi: 10.1186/1744-_9081-_2-_3.